

Some "concepts"
(see also Physical polymer science by L.H. Sperling)
Molecule - Molecular Weight of a molecule is fixed (benzene = 78g/mol)
Oligomer - 10-100 repeat units. (if each mer was 50g/mol then it is 500-5000g/mol)
Polymer - Molecular weight 25,000-1,000,000g/mol. (if each mer was 50g/mol then it is 500-20,000 repeat units). 25,000g/mol is usually also close to the onset of entanglement.
- Molecular Weight of a polymer depends on the method of preparation. Also, they are polydisperse (meaning the sample contains more then one species).
- Number average molecular weight:

- Weight average molecular weight:
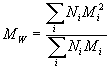 == Ni the number of molecules of molecular weight Mi.
== Ni the number of molecules of molecular weight Mi.
- Conformation - spatial arrangement. For a polymer it depends on: temperature, solvent, structure, crystallisation, extension, presence of other species (polymers).
- Dissolving a polymer which is below its Tg-
Stage 1 = solvent wets the polymer
Stage 2 = swelling (case II swelling that doesn't follow diffusion law)
Stage 2b = polymer swells à Tg is lowered. Swells until Tg below ambient (Ta).
Stage 3 = Solvent diffuse into the polymer parts (diffusion or Fick's law) that satisfy (Tg<Ta). This is rapid.
Stage 4 = Polymer diffuse into the solvent.
(Cross-linked polymer only undergoes stage 1 and stage 2).





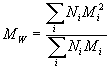 == Ni the number of molecules of molecular weight Mi.
== Ni the number of molecules of molecular weight Mi.